Which of the Following Are Double-displacement Reactions
You may want to consult a list of Ksp values to solve these problems. Start studying 507 double displacement reactions.

Chemical Reactions Types Definitions And Examples Teaching Chemistry Chemistry Classroom Chemistry Notes
Double displacement reactions have.

. This reaction is also called oxidation-reduction reaction. Double displacement reactions involving acid bases are called a neutralisation reaction. A redox reaction is a type of reaction which involves transfer of electrons among two species.
In oxidation process there is an increase in oxidation state of an atom ion or molecule by losing the electrons. You may want to consult a list of K sp values to solve these problems. 2NaCl CaSO 4 Na 2 SO 4 CaCl 2.
A single replacement reaction also known as a single displacement reaction occurs when one element in a molecule is swapped for another. Which of the following equations represents a decomposition reaction. A double displacement reaction is a reaction in which the cation of a reactant displaces the cation of the other reactant and vice versa.
A double displacement reaction is the one in which the two reacting species exchange their ions. Double replacement reactions also called double displacement exchange or metathesis reactions occur when parts of two ionic compounds are exchanged making two new compounds. AgNO 3 HCl AgCl HNO 3.
Will this reaction occur. Examples of double-displacement reactions are Redox reactions or neutralization reactions. NaOH aq HCl aq NaCl aq HOH l.
So it is a double displacement reaction. These are many types like precipitation reaction neutralization reaction etc. Ii is an example of a double displacement reaction because both of the reacting compounds are exchanging ions.
A double displacement reaction would be. A double replacement reaction is represented by the general equation. H 2 SO 4 2LiOH Li 2 SO 4 2H 2 O.
A HCI 9 - Hz g Cl2 9 B HCI aq NaOH aq - H2O 1 Naci aq C Mg 2HCl aq - MgCl2 aq H2 g D HCI 9 C-H10 9 -CsHCI 9 1. View the full answer. I is an example of a single displacement reaction as only one ion is being exchanged.
Letter B is an example of a double displacement reaction because Barium displaces Hydrogen and vice versa. So there are two. Indicate which of the following double displacement reactions will go to completion and circle what the product of the reaction will be if any.
The reactions in which two compounds react by an exchange of ions to form two new compounds are called double displacement reactions. The reaction 2C₂H₂ g 5O₂ g -- 4CO₂ g 2H₂O l is. An example of a double displacement reaction is the reaction between sodium hydroxide and hydrochloric acid to for sodium chloride as shown.
2HgO s -- 2Hg l O₂ g The reaction Pb NO₃₂ aq 2KI aq -- PbI₂ s 2KNO₃ aq is a. 6NaCl Fe2CO33 As you can see the sodium has replaced the iron AND the iron has replaced the sodium. 3Na2CO3 2FeCl3 --.
During this reaction the cations and anions of two different compounds switch places forming two entirely different compounds. HCl NaOH NaCl H 2 O. Which of the following reactions is a double displacement reaction.
In the reaction between ferric chloride and sodium hydroxide there is exchange of Cl and OH ions between the reactants to form ferric hydroxide and sodium chloride. Chemistry Questions Answers for AIEEEBank Exams. Here are some examples of double displacement reaction.
Double Displacement Reactions Indicate which of the following double displacement reactions will go to completion and circle what the product of the reaction will be if any. B Reaction of hydrochloric acid with sodium hydroxide. A double displacement reaction involves exchange of ions between the reactants in order to form the products.
D All of the above Explanation. Precipitation and Double-Displacement Precipitation reactions belong to a general class of reactions called double-displacement reactions. Learn vocabulary terms and more with flashcards games and other study tools.
Therefore it is a double displacement reaction. Which of the following is NOT a double displacement reaction. In double replacement reactions the positive ions exchange negative ion partners.
Which of the following reactions is a double displacement reaction. The two compounds exchange their ions to form sodium chloride and water. In a double displacement reaction atoms from two.
Eg2K 2H_2O rightarrow 2KOH H. Mg2 SO4-2 2 Na1 2 OH-1 Nit2 c1 2 Na1 2 Br1 Ag1N03-1 Na11-1 Pb2 2 NO3-1 2 Na1 2 Br-1-. Correct option is C Double displacement reactions may be defined as the chemical reactions in which one component each of both the reacting molecules is exchanged to form the products.
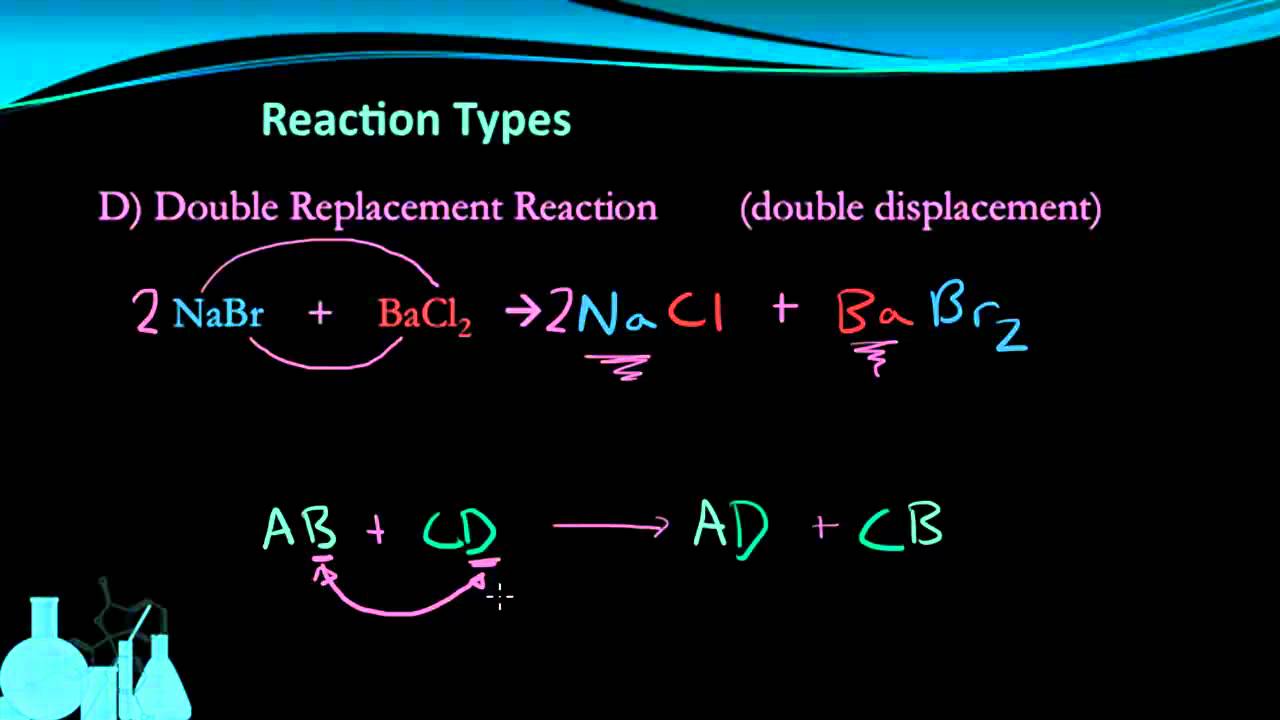
AB CD AD CB. A HCI g H2 g Cl2 g B HCI aq NaOH aq H2O 1 NaCl aq C Mg s 2HCl aq MgCl2 aq H2 g D HCI g. Pb NO 3 2 2NaCl 2NaNO 3 PbCl 2.
Answer Explanation Answer. Al 2 SO 4 3 6NH 4 OH 2Al OH 3 3 NH 4 2 SO 4. The overall pattern of a double replacement reaction looks like this.
Aqueous hydrochloric acid mixed with an aqueous solution of sodium hydroxide. A metathesis reaction is also called a double displacement reaction is a chemical process involving the exchange of ions of cations and anions between reacting species resulting in the creation of two new chemical species. Lets look at all the options.
Which of the following double displacement reactions will occur complete the equations that do. AgNO 3 NaCl AgCl NaNO 3. Will this reaction occur.

Chemical Reactions Periodic Table Ios App Chemistry Chemical Reactions Periodic Table

You Ll Need To Recognize Each Of These Reactions For The Teas Science Section Chemistry Worksheets Chemistry Lessons Teaching Chemistry

Chemical Reaction Type Chart Reaction Types Chemical Reactions Teaching Chemistry

A Double Replacement Reaction Is A Chemical Reaction Where Two Reactant Ionic Compounds Exchange Ions To Form Two New Product Compounds With The Same Ions

Synthesis Reactions Formation Reactions Module 5 Chemical Reactions Reactions Synthesis

Chemistry Redox Reaction Youtube Redox Reactions Chemistry Reactions

There Are 4 Main Types Of Chemical Reactions Synthesis Decomposition Single Displacement And Double Disp Chemistry Lessons Chemical Reactions Reaction Types

Predicting Products Of Synthesis And Decomposition Reactions Teaching Chemistry Scientific Method Lesson Writing Linear Equations

Chemical Reactions Allow Humans To Create Fundamental Things Out Of Raw Ingredients Such As Orange Chemical Reactions Interactive Notes Earth And Space Science

Types Of Chemical Reactions Chemical Reactions Chemical Equation Chemical

Predicting The Products Of Single And Double Displacement Reactions Teaching Chemistry Chemical Equation Interactive Science Notebook

Double Replacement Reaction Definition And Examples Reactions Covalent Bonding Ap Chemistry

Double Displacement Reaction Chemical Reactions Chemical Changes Chemical And Physical Changes
Chemistry Module 5 Types Of Chemical Reactions Http Www Youtube Com Watch V Aawccqb75d0 Single

Chemistry Lesson 35 Double Displacement Reactions Chemistry Lessons Chemistry Lesson

Chemical Reactions Chemical Reactions Occur In Predictable Patterns Chemical Reactions Chemical Changes Chemical

Introduction To Chemical Reactions Chemistry Lessons Teaching Chemistry Chemistry

Image Result For Double Displacement Reaction Science Experiments Physics Notes

Comments
Post a Comment